
Ever wonder why some drugs have two different names? One sounds like a science experiment - albuterol - and another like a word from a different language - salbutamol. They’re the same drug. And that’s not a mistake. It’s the result of two global systems trying to keep patients safe while working in slightly different languages.
Why Generic Drug Names Even Exist
Pharmaceutical companies don’t just invent drugs. They invent names too. But here’s the catch: once a drug is approved, it can’t be sold under just one company’s brand name forever. Generic versions have to be allowed. That’s where nonproprietary names come in - the official, public-domain names everyone uses when they’re not talking about the brand. These names aren’t chosen for marketing. They’re chosen for safety. A name like lipitor might sound nice, but if a doctor writes lipitor and a pharmacist misreads it as lipitor vs. lipitor (a real example of how similar names cause errors), someone could get the wrong dose. That’s why the system is built to make names as clear, unique, and predictable as possible.USAN: The U.S. Standard
In the United States, the United States Adopted Names (USAN) program decides what generic names are used. It’s been around since 1964 and is run by a council made up of the American Medical Association, the U.S. Pharmacopeia, and the American Pharmacists Association. Their job? Make sure every new drug has a name that’s easy to say, spell, and remember - and that doesn’t sound like any other drug already on the market. USAN names follow a strict pattern. Most end in a stem - a suffix that tells you what kind of drug it is. For example:- -prazole = proton pump inhibitors (omeprazole, pantoprazole)
- -statin = cholesterol-lowering drugs (atorvastatin, rosuvastatin)
- -mab = monoclonal antibodies (rituximab, adalimumab)
INN: The Global Standard
While USAN handles the U.S., the International Nonproprietary Names (INN) program, run by the World Health Organization since 1950, does the same thing worldwide. INN names are meant to be used everywhere - from Brazil to Bangladesh. Here’s the surprising part: USAN and INN agree on about 95% of names. But when they don’t? That’s where confusion happens.- Acetaminophen (USAN) = Paracetamol (INN)
- Albuterol (USAN) = Salbutamol (INN)
- Rifampin (USAN) = Rifampicin (INN)

How Brand Names Fit In
Brand names are different. They’re not about safety. They’re about marketing. Think Advil, Lyrica, Humira. These names are designed to be catchy, memorable, and trademarkable. They can be anything - even made-up words like Viagra or Prozac. But here’s the rule: a brand name can’t sound too much like the generic name. You can’t call your version of omeprazole “Omeprazolix.” That would confuse doctors and pharmacists. The FDA and WHO both require brand names to be distinct enough to avoid mix-ups. Some companies try to make brand names hint at the generic. Eliquis sounds like “apixaban” - the generic. That’s intentional. It helps with recall. But it’s risky. If the brand name becomes too similar, regulators will reject it.Why the Stem System Matters
The real genius of USAN and INN isn’t just the names themselves - it’s the pattern. When a doctor sees a drug ending in -virdine, they know it’s an HIV drug. If it ends in -dipine, it’s a calcium channel blocker for blood pressure. That’s not coincidence. It’s design. Pharmacists can spot drug classes at a glance. Nurses can verify prescriptions faster. Patients get fewer errors. A 2022 study in the Journal of Clinical Pharmacology pointed out that as drugs get more complex - like gene therapies or RNA-based treatments - the old stem system struggles. What’s the stem for a drug that edits your DNA? There isn’t one yet. That’s why both USAN and WHO are working on new frameworks. But they’re cautious. Change too fast, and you risk confusion.The Hidden Cost of Bad Names
Medication errors due to similar-sounding names cost the U.S. healthcare system about $2.4 billion a year. That’s not just money. It’s hospital stays, missed work, and sometimes lives. One real case: a patient was given zolpidem (a sleep aid) instead of zopiclone (a similar sedative). The mix-up led to a fall and broken hip. Both drugs end in “-em” and “-one.” Easy to confuse. That’s why USAN and INN now test names using phonetic software and human testing - even checking how names sound in accents from different regions.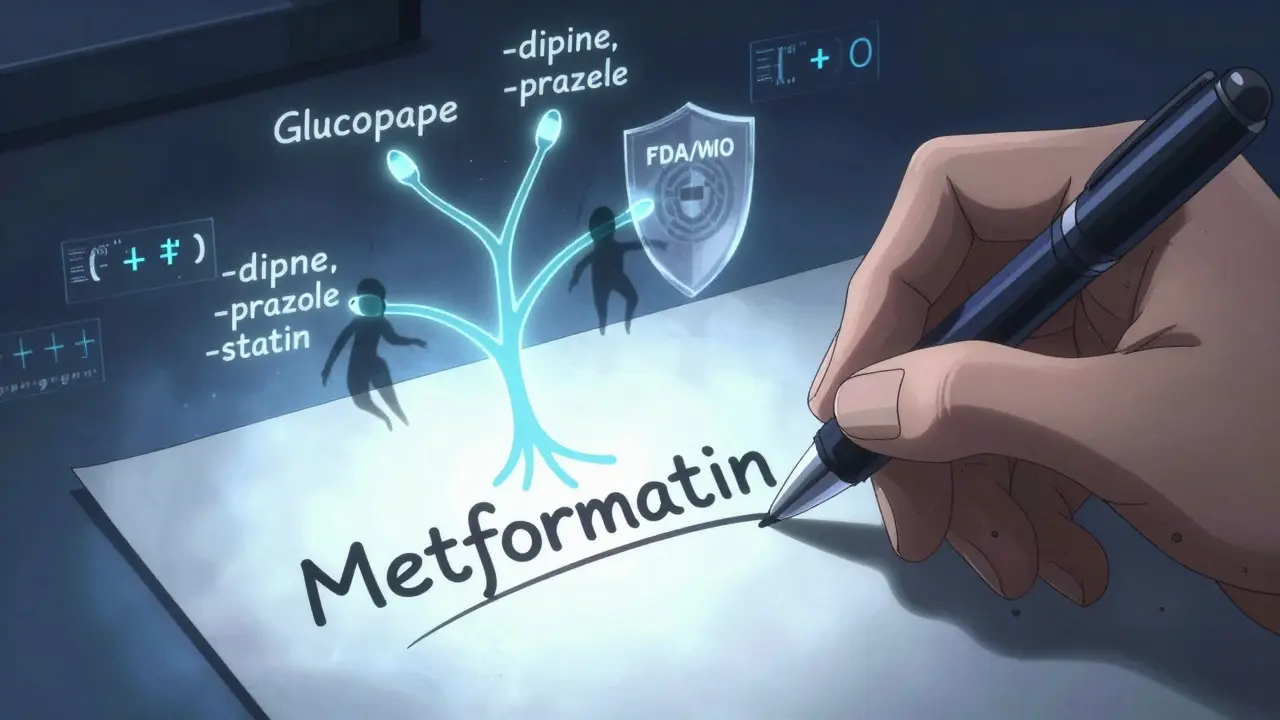
What Happens When a Drug Fails
Not every drug makes it to market. About 65% of drugs that get a USAN name never reach pharmacies. Why? They fail in clinical trials. But their names stay on record. Why keep them? Because if another company later develops a similar drug, they can’t reuse that name. The system is designed to be permanent - even for drugs that never exist. Meanwhile, the INN program accepts names even if the drug is only approved in one country. That’s why you’ll see INN names on packaging in Europe, even if the drug is still under review in the U.S.What’s Next for Drug Naming
The future of drug naming is about flexibility. New therapies - like CRISPR-based treatments or personalized cancer vaccines - don’t fit into old categories. There’s no “-gene” or “-vax” stem yet. But the USAN and WHO teams are already talking about it. They’re also pushing for more alignment. The gap between USAN and INN is shrinking. New drugs are more likely to get the same name globally. But exceptions like acetaminophen/paracetamol will likely stick around - because changing them now would cause more confusion than keeping them. The bottom line? Drug names aren’t random. They’re carefully built tools. Every syllable is checked. Every letter is weighed. And behind every name is a system designed to keep you safe - whether you’re in New York, Nairobi, or Manchester.Why You Should Care
If you take any prescription, you’re using this system. When your pharmacist says, “This is the generic version of your brand drug,” they’re using the USAN or INN name. When your doctor writes a script, they’re relying on the fact that metformin means the same thing everywhere. It’s not glamorous. But if you’ve ever avoided a medication error because a name was clear - that’s this system working.What’s the difference between a generic name and a brand name?
The generic name (like metformin or lisinopril) is the official, public-domain name used by all manufacturers and regulators. It’s standardized by systems like USAN and INN. The brand name (like Glucophage or Zestril) is created by a single company for marketing and is trademarked. Only that company can use it - until the patent expires, then others can sell the same drug under the generic name.
Why does the same drug have different names in the U.S. and Europe?
It’s because the U.S. uses USAN and most other countries use INN. While they agree on most names, a few differ due to historical usage, pronunciation preferences, or regulatory choices. Examples include albuterol (U.S.) vs. salbutamol (Europe) and acetaminophen (U.S.) vs. paracetamol (Europe). These differences can cause confusion, especially for travelers or international prescriptions.
How are drug names chosen?
Drug companies propose up to six names during early clinical trials. Experts at USAN and INN review them for conflicts with existing names, pronunciation, spelling, and potential for confusion. They look at stems to identify drug class, check for trademark issues, and even test how names sound in different languages. Only after multiple rounds of feedback and revision is a name approved - a process that takes 18-24 months.
Do all drugs need a generic name?
Yes. All new drug substances, whether they’re pills, injections, or biologics, must have a nonproprietary name approved by either USAN (in the U.S.) or INN (globally). This is required by law for regulatory approval. Even experimental drugs get a name before they’re tested in humans.
Can a brand name become a generic name?
Sometimes, but not officially. Brand names like aspirin or heroin became so popular that people started using them as generic terms. But legally, they’re still trademarks. Companies fight to prevent this - because if a brand becomes generic, they lose their trademark rights. That’s why you’ll see ads saying, “This is the brand name for ibuprofen,” to remind people it’s not the generic term.
Write a comment
Your email address will not be published.




14 Comments
So let me get this straight - we spend millions to make sure ‘albuterol’ doesn’t look like ‘salbutamol’… but people still mix up ‘Zoloft’ and ‘Zyprexa’? 🤦♂️ This system works… until someone’s handwriting looks like a toddler drew it.
Y’all act like this naming thing is some sacred science. Nah. It’s corporate bureaucracy with a lab coat. USAN and INN are just two old men in suits arguing over whether ‘-mab’ should be ‘-mab’ or ‘-mab-1’ while the real winners are the pharma CEOs who get to patent ‘Fluffinex’ for $200 a pill.
It’s funny how we treat drug names like they’re poetry - every syllable weighed, every stem chosen with divine care - but we let the same people name entire cities after themselves. If ‘Humira’ can be trademarked, why can’t ‘New York’ be called ‘Elonville’? The system’s inconsistent. And honestly? I kind of love that.
Wait… so ‘acetaminophen’ and ‘paracetamol’ are the SAME THING?? I’ve been buying both in different countries thinking I was getting different meds?? I’m not even mad… I’m impressed. And also terrified. Someone please tell me my liver is okay.
Why does America even have its own naming system? We’re not special. We just like being difficult. Nigeria uses INN. So does India. So does Brazil. But nooo, the US has to invent ‘USAN’ like it’s a new religion. This isn’t innovation - it’s nationalism disguised as safety
Stems are genius. If you see ‘-prazole’, you know it’s a stomach drug. If you see ‘-tidine’, it’s an H2 blocker. It’s like a secret language for med pros. I wish we had this for car parts.
Let’s be real - the stem system is collapsing under biologics. Antibody-drug conjugates? Bispecifics? The current nomenclature can’t handle it. We’re patching a 1970s OS with 2024 code. Someone needs to architect a new taxonomy before a nurse accidentally gives a patient a CRISPR therapy labeled ‘-vax’.
This is actually kind of beautiful? 🤯 Like, someone sat down and thought: ‘How do we prevent people from dying because two names sound like ‘blarg’ and ‘blargh’?’ That’s not just bureaucracy - that’s love. 💙
And yet, people still get the wrong drug. So all this ‘science’ is just theater. If it worked, we wouldn’t have 2.4 billion in errors. It’s a band-aid on a bullet wound. And someone’s making money off the band-aid.
So if a drug fails clinical trials, its name stays dead but registered? That’s wild. Like a ghost name haunting the database. I wonder if any of them have names that sound like they could’ve been hit songs.
Actually, I think this system is one of the most underappreciated public health wins. No one talks about it, but it prevents thousands of errors a year. The fact that it’s so quiet means it’s working.
Think about this: every time you take a pill, you’re trusting a naming system that’s been refined for 70 years by teams of pharmacists, linguists, and lawyers. That’s not magic. That’s community. And it’s worth protecting.
As someone who’s had to explain ‘paracetamol’ to an American cousin who thought it was ‘something Nigerian’… I can tell you - this naming divide isn’t just bureaucratic. It’s cultural. And honestly? I kinda miss when we all just called it ‘painkiller’.
USAN is just American arrogance with a thesaurus