
It’s 2025, and you walk into your local pharmacy asking for the generic version of your expensive prescription. The pharmacist shakes their head. "It’s been approved by the FDA since last November," they say. "But we still can’t get it." This isn’t rare. It’s the new normal.
Why Approved Doesn’t Mean Available
The FDA approved 63 first generics in 2025. That sounds like progress. But here’s the catch: the average time between FDA approval and actual market availability is now 3.2 years. That’s not a typo. That’s the reality for hundreds of drugs. Even when a generic drug passes every safety and quality check, it can sit on a shelf-legally blocked-from reaching patients. The main reason? Patent litigation. Brand-name drug companies file lawsuits against generic manufacturers the moment they file for approval under a Paragraph IV certification. This triggers a 30-month automatic stay, halting the FDA from giving final approval. It doesn’t matter if the patent is weak, outdated, or even questionable. The clock starts anyway. And in 2024, 68% of all generic applications included this kind of patent challenge.The Patent Thicket Problem
It’s not just one patent anymore. It’s dozens. The average number of patents listed per brand drug in the FDA’s Orange Book jumped from 12.3 in 2020 to 14.7 in 2025. Some drugs, like AbbVie’s Humira, have been wrapped in over 200 patents. These aren’t all groundbreaking inventions. Many are minor tweaks-new dosages, packaging, or delivery methods-designed to extend exclusivity. This tactic, called patent thicketing, has become standard practice. Dr. Aaron Kesselheim from Harvard Medical School put it bluntly: “The current patent thicketing strategies have extended monopolies beyond the intended 20-year term by an average of 3.7 years per drug.” That’s over three years of patients paying full price because of legal maneuvering, not innovation.Who Gets Hit the Hardest?
Not all drugs face the same delays. Complex generics-like injectables, inhalers, and biosimilars-are the worst off. For oncology drugs, the average delay between approval and launch is 4.1 years. Cardiovascular generics? 2.8 years. Simple oral pills? Closer to 2.3 years. Why? Because complex drugs are harder to copy. They require more testing. And brand companies know that. So they pile on more patents, knowing it will take longer-and cost more-for a generic maker to fight back. Small generic companies are especially vulnerable. In 63% of delayed cases, the manufacturer earns less than $500 million a year. Legal fees for a single patent case now average $12.7 million. For a small firm, that’s a gamble they can’t afford. Many just walk away.
The Supply Chain Complication
Patents aren’t the only problem. Supply chain issues are making things worse. In 37% of delayed generic launches between 2023 and 2025, the issue wasn’t legal-it was missing active ingredients. Especially for injectables, the API (active pharmaceutical ingredient) often comes from just one or two overseas suppliers. If there’s a disruption-political, logistical, or quality-related-the whole launch gets pushed back. Companies like Teva and Sandoz are trying to fix this. In 2022, they had an average of 1.8 approved API suppliers. By 2025, that number rose to 3.4. More suppliers mean less risk. But it takes years to build those relationships. Most generics still operate on thin margins. Diversifying supply isn’t cheap.What’s the FDA Doing?
The FDA has no power to override patent lawsuits. That’s the law. But they’ve tried to help. Their AI-assisted review system, rolled out in early 2025, cut approval times by 22% for non-litigated applications. That’s good news-for the 28% of generics that aren’t caught in court. They’ve also started cleaning up the Orange Book. In May 2025, FDA Director Dr. Patrizia Cavazzoni admitted that improper patent listings have been used to block competition unfairly. The agency is now pushing for stricter rules on what patents can be listed. No more “method of use” patents for drugs already off-patent. No more listing patents that don’t actually cover the drug. But change is slow. The FDA’s Citizen Petition process-meant to challenge bad patents-now takes an average of 18.2 months to resolve. That’s longer than the review time for most drugs.Patients Are Paying the Price
Real people are suffering. A September 2025 survey by the Association for Accessible Medicines found that 82% of pharmacists get asked daily about approved but unavailable generics. The top drugs? Eliquis, Trulicity, Steglatro-each costing over $400 a month. The generic version? Around $85. Patients For Affordable Drugs Now documented 412 cases between 2023 and 2025 where people skipped doses or stopped treatment because they couldn’t afford the brand. One man with diabetes rationed his insulin. A woman with rheumatoid arthritis used leftover samples from two years ago. These aren’t outliers. They’re symptoms of a broken system. Hospital pharmacies report that patent delays are a leading cause of drug shortages. In 78% of cases, pharmacy directors say they’re forced to use more expensive alternatives-or go without.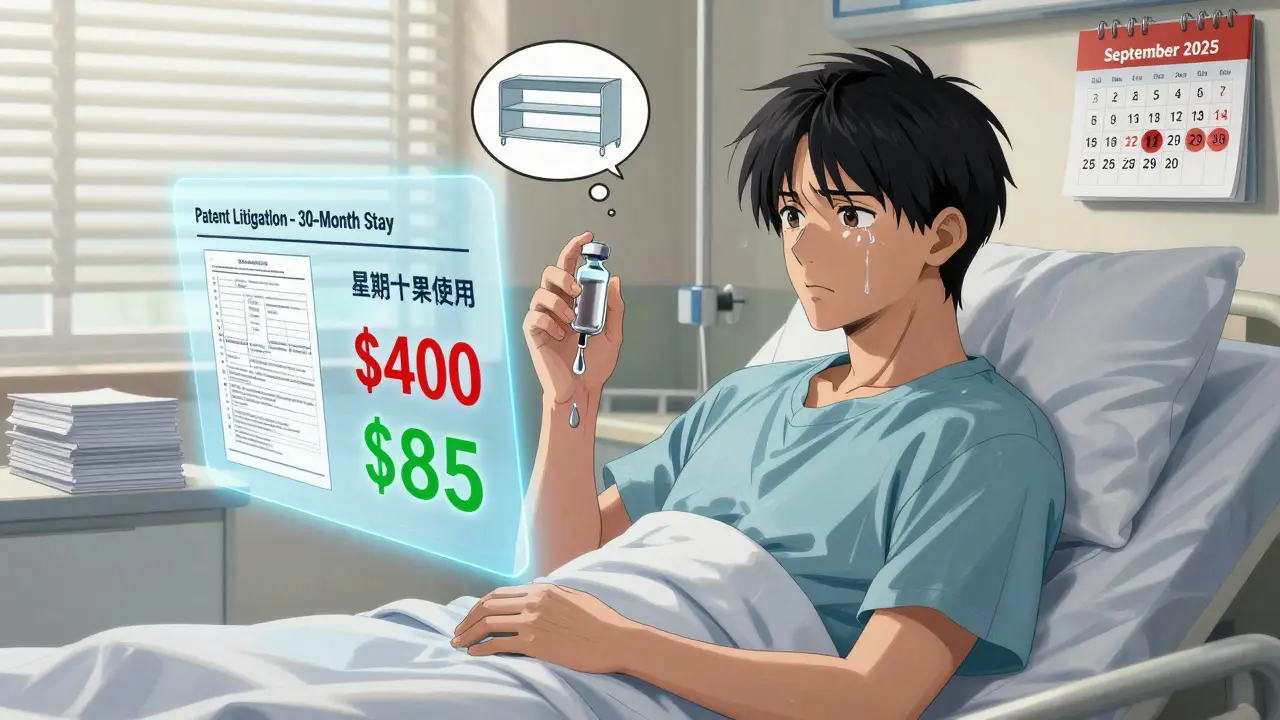
Is There a Solution?
There are proposals. The CREATES Act, introduced in Congress in 2025, would force brand companies to provide samples to generic makers for testing. Right now, many refuse, claiming safety concerns. That’s a legal loophole that delays development by years. The FTC has stepped in too. They’ve filed seven enforcement actions against companies using patent tactics to block competition. One case against Jazz Pharmaceuticals over Xyrem led to an agreement that let generics enter the market months earlier than planned. But the biggest fix? Limiting how many patents a company can list per drug. Right now, there’s no cap. McKinsey found that 67% of industry stakeholders support a limit. The pharmaceutical lobby, PhRMA, says that would hurt innovation. But if the goal is to reward real innovation-not legal tricks-then change is overdue.The Bigger Picture
The U.S. spends $3.2 billion extra every year on brand-name drugs that could be generic, thanks to patent delays. That’s Medicare money. That’s taxpayer money. That’s money families lose out on. Meanwhile, Europe moves faster. The average time from approval to market there? Just 1.7 years. Why? Because their patent system doesn’t let lawsuits automatically pause everything. They resolve disputes faster. They don’t let patent thickets go unchecked. The U.S. system was built to balance innovation and access. But today, it’s tilted hard toward protectionism. The 30-month stay was meant to be a pause, not a permanent wall. Now, it’s being used to delay competition for years-even when the patent is clearly invalid.What Comes Next?
The top 10 drugs losing exclusivity in 2025 represent $78.3 billion in annual sales. That’s a massive opportunity for generics. But without reform, most of that money will stay with brand companies. The new FDA Commissioner, Dr. Peter Bach, has signaled he wants more transparency. If he follows through-by forcing faster Orange Book updates, cracking down on bad patents, and supporting the CREATES Act-generic entry could speed up by 8 to 12 months by 2027. But it won’t happen on its own. Patients, pharmacists, and providers need to keep asking: Why is this drug approved but not here? Why are we paying more because of a legal delay? The system won’t fix itself. Someone has to push it.Write a comment
Your email address will not be published.





13 Comments
The FDA approving generics but they still don’t reach shelves is a scam wrapped in bureaucracy. Patients are dying while lawyers get rich. This isn’t innovation-it’s extortion.
Every time I fill a script for someone on insulin, I see the fear in their eyes. They know the price drop should be happening. It’s not. And no one’s holding anyone accountable.
It’s wild how we’ve turned pharmaceuticals into a courtroom sport. In Europe, they don’t let patent trolls hijack access like this. Here, we’ve got a system that rewards delay as a business model. The 30-month stay was supposed to be a buffer, not a fortress. Now it’s a velvet rope for Big Pharma’s VIP lounge.
And don’t get me started on the Orange Book-half those patents shouldn’t even be listed. It’s like listing the color of the pill bottle as intellectual property.
Meanwhile, patients are cutting pills in half. That’s not healthcare. That’s tragedy dressed in corporate jargon.
I work in a rural clinic and this is real life every day
One lady came in crying because her arthritis med was approved but not in stock and she couldn’t afford the brand
I told her I’d call every pharmacy in the county and still didn’t find it
It’s not a policy problem it’s a humanity problem
I think people don’t realize how much this affects families. My dad’s on a med that’s been approved for 18 months but still costs $500 a month. He’s on a fixed income. He’s not lazy. He’s not greedy. He’s just trying to survive.
And the worst part? No one seems to care until it’s their turn.
Why is it so hard to just let people get cheaper medicine when the science says it’s safe?
It’s not rocket science. It’s just greed.
Let’s be real for a second. The whole system is a rigged game where patent lawyers are the real innovators. You don’t need a lab coat to make money anymore-you need a law degree and a copy of the Orange Book.
They’re not blocking generics because they’re protecting innovation. They’re blocking them because they’ve figured out how to turn the legal system into a cash register.
And the worst part? It’s legal. That’s the real horror story. The law isn’t broken-it was designed this way.
Every time you hear about a drug being approved but not available, just remember: someone’s making millions off your suffering, and they’re doing it with a straight face and a perfectly worded brief.
It’s not a glitch. It’s the business model.
And until we stop treating medicine like a commodity and start treating it like a right, nothing will change.
Meanwhile, we’re all just waiting for the next $400 pill to get stuck in legal purgatory.
And the FDA? They’re stuck playing whack-a-mole with patents that shouldn’t even exist.
They’re not powerless-they’re just choosing not to use the power they have.
They could clean up the Orange Book tomorrow. They could reject patent listings that are clearly absurd. But they don’t. Why? Because the lobbying money is too good.
So we’re left with a system where a drug can pass every safety test but still be denied to patients because a company owns a patent on the shape of the tablet.
That’s not progress. That’s madness.
And we’re all just supposed to shrug and pay more.
Good luck getting your insulin next month. I hope your bank account is ready.
Why is the FDA letting foreign countries control our medicine supply chain
China and India are holding our lifesaving drugs hostage
And now they want to give generics to people who dont even pay taxes
THIS IS A WAR ON AMERICA
They want us to be weak and dependent
They dont care if we die
They just want to control us
Wake up people
Let’s cut the fluff. The U.S. generic market is a joke. The delays aren’t accidental-they’re engineered. Big Pharma pays off politicians, the FDA’s hands are tied by lobbyists, and the CREATES Act? A toothless bill with a fancy name.
Meanwhile, Australia and Canada are laughing all the way to the pharmacy counter.
And the worst part? Nobody in Congress has the balls to fix it. Why? Because their donors are the same CEOs who profit from these delays.
It’s not a broken system. It’s a perfectly optimized one-for profit, not patients.
And if you think the supply chain issue is the real problem, you’re missing the forest for the trees. The patents are the root. The supply chain? Just the convenient excuse.
Also, Teva and Sandoz? They’re not heroes. They’re just the biggest players who can afford to play the game. Small companies? They’re cannon fodder.
So yeah. We’re all just pawns in a game where the board was rigged from the start.
They approved it
But they won’t let you have it
That’s not healthcare
That’s a horror movie
And we’re all just watching
Waiting for the next name to be added to the list
Of people who died because a patent was too shiny
Too clever
Too legal
I’ve been reading about this for months and I keep wondering-how many people have given up because they just stopped asking?
Not because they’re okay with paying $400
But because they’re tired of hearing "it’s approved" and then being told "we don’t have it"
What does that do to someone’s trust in the system?
I think we’re normalizing suffering here.
And no one’s talking about it.
It’s heartbreaking but I’m so glad someone’s finally putting this out there 💔
My sister had to skip her heart med for two months because the generic wasn’t available
She’s fine now but I’ll never forget the look on her face when she said "I just didn’t want to be a burden"
We need to fix this. Like, yesterday.
❤️✊
It’s a textbook case of regulatory capture and rent-seeking behavior masquerading as IP protection. The 30-month stay, when weaponized via Paragraph IV challenges, creates a classic hold-up problem in the context of asymmetric information and high sunk costs for generic entrants.
Moreover, the proliferation of patent thickets-particularly in biologics and complex generics-exacerbates the entry deterrence effect, effectively extending monopolistic pricing power beyond statutory limits.
The FDA’s AI-driven review system is a band-aid on a hemorrhage. Structural reform-namely, capping Orange Book listings and eliminating automatic stays-is the only viable path forward.
Without it, we’re just redistributing wealth from patients to shareholders under the guise of innovation.
I just want to say thank you to everyone who’s been speaking up about this.
It’s easy to feel powerless when you’re just trying to get your meds.
But the fact that we’re talking about it-that pharmacists are sharing stories, patients are speaking out, and people are demanding change-that’s how things start to shift.
We don’t need to agree on everything.
But we can all agree that no one should have to choose between their health and their rent.
Keep going. We’re not alone.
Actually, the FTC’s enforcement actions are more meaningful than most realize. The Jazz Pharmaceuticals case set a precedent-when you force a company to provide samples under antitrust law, you’re not just opening the door for generics. You’re dismantling the entire infrastructure of delay.
That’s the real leverage point.
Not the FDA’s slow cleanup.
Not the CREATES Act’s vague language.
But the antitrust hammer.
And if they keep using it, we might actually see a drop in delays by 2027.
It’s not perfect. But it’s the only tool that actually hurts the right people.